Russia registers its second COVID-19 vaccine
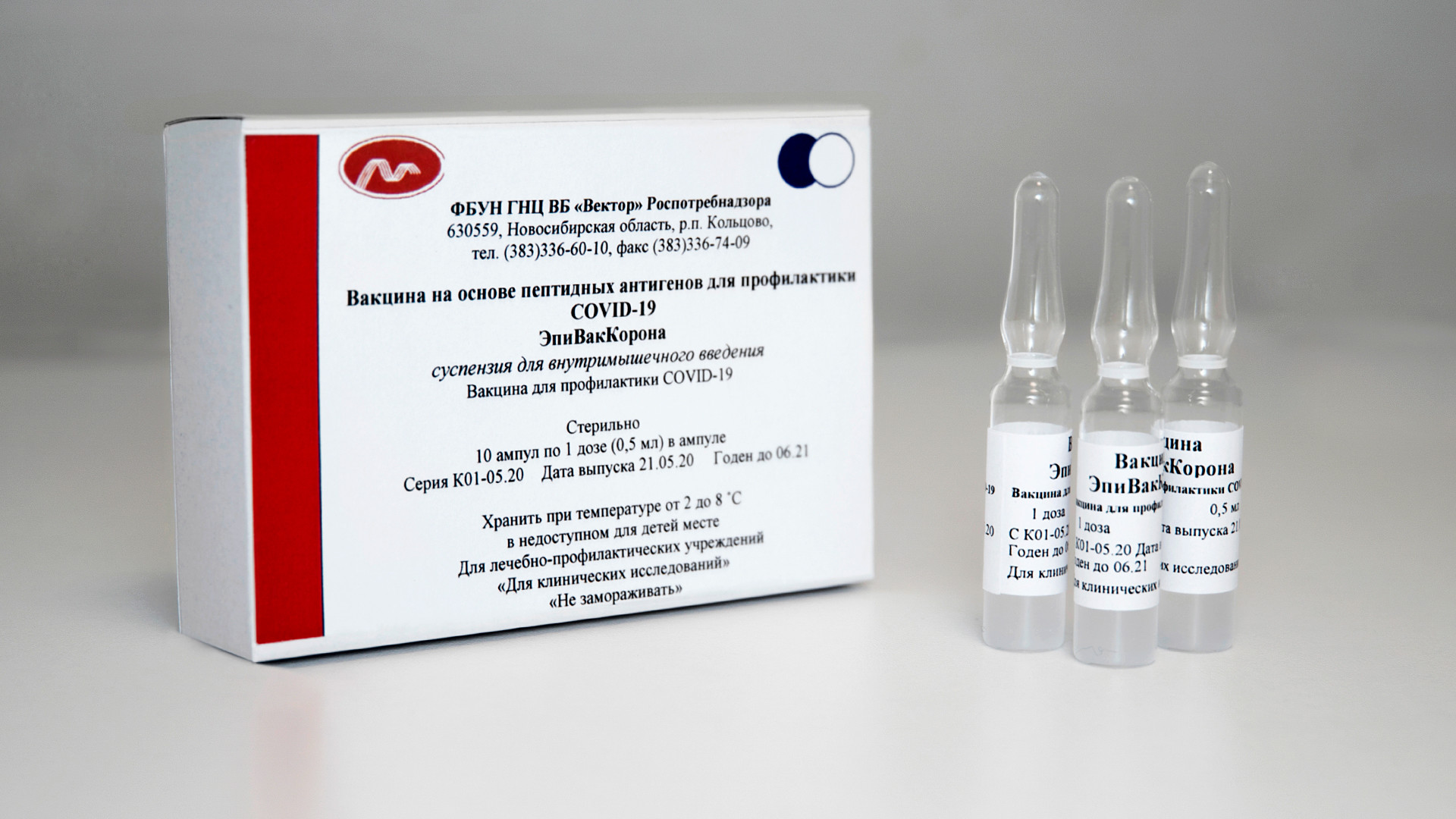
The vaccine for coronavirus, EpiVakKorona developed by the State research center of Virology and biotechnology "Vector"
SputnikOn October 14, President Vladimir Putin announced the registration of a second vaccine against the coronavirus. The announcement was made during a cabinet meeting.
“I would like to begin with some pleasant news regarding the Novosibirsk-based ‘Vektor’ laboratory registering today the second vaccine against the coronavirus - EpiVakKorona,” Putin said.
Deputy Prime Minister Tatyana Golikova clarified that the vaccine is different from ‘Sputnik-V’, registered earlier in August 2020. In order to create the first vaccine, scientists at the Gamaleya National Center of Epidemiology and Microbiology used a carrier virus, which delivers the coronavirus’s DNA into the organism, eliciting an immune response. These are known as vector-based vaccines.
READ MORE: What we know about the FIRST registered COVID-19 vaccine in the world
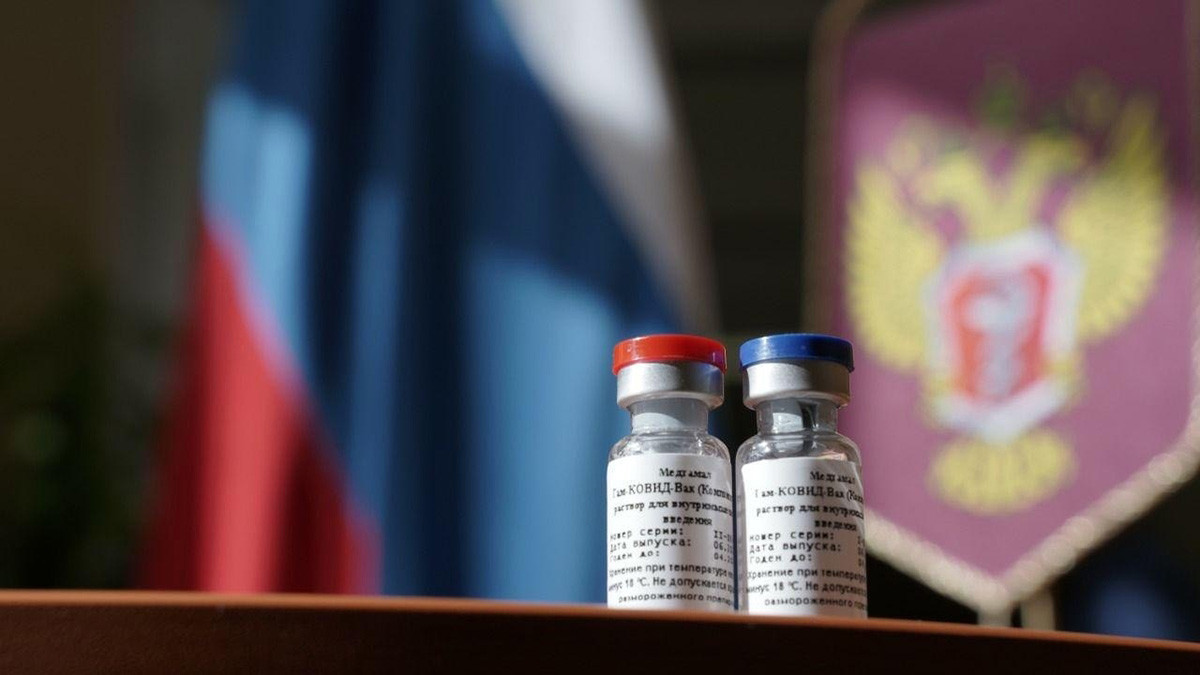
A vaccine against a new coronavirus infection was registered in Russia for the first time in the world on August 11, 2020- - -Sputnik
SputnikEpiVakKorona consists not of the carrier virus itself, but from a synthetic network of short peptide proteins - through them “the immune system learns to recognize and neutralize the virus,” Golikova said. The same type of vaccine was created during the Ebola pandemic, also by the Gamaleya Center.
Prior to registration, the vaccine underwent two trial phases, Vektor director Rinat Maksyutov explains. The first clinical trial included 14 subjects who were made aware of what they were being injected with. The second test consisted of 86 volunteers, 43 of whom received the new vaccine, while the second half received a placebo.
“The research participants are feeling well. Two had developed a slightly painful sensation at the site of injection within 24 hours. This was to be expected, as the vaccine contains aluminum hydroxide,” Maksyutov said of the side effects.
The first 60,000 doses will be released in the near future, before Vektor starts clinical trials across Russia. There are 40,000 participants overall, including 150 persons older than 60, considered to be in the at-risk group.
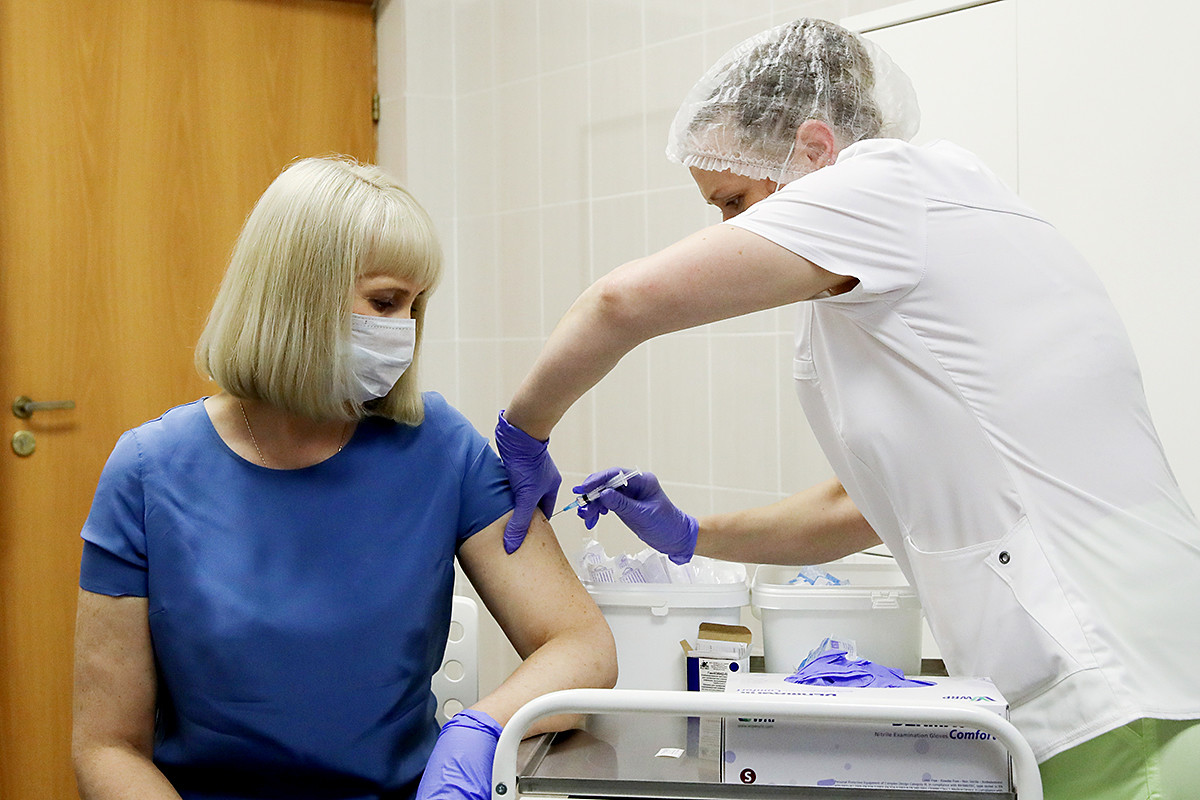
Volunteers take part in post - registration trials of the Russian COVID-19 vaccine
Sergey Bobylev/TASSThe vaccine should become available on January 1, 2021, according to the government’s drug database website.
Meanwhile, the Sputnik-V trials involved 13,000 people, Golikova said.
READ MORE: What we know about the Sputnik V vaccine as reported in The Lancet (BRIEF EXPLAINER)
A third vaccine is currently undergoing its first phase of clinical trials at the The Chumakov Federal Scientific Center for Research and Development of Immune and Biological Products, where it was created. According to the General Director Aydar Ishukhametov, the vaccine can potentially create immunity to COVID-19 for decades to come.
READ MORE: Russia has several working COVID-19 vaccines. How safe are they? (DETAILED EXPLAINER)
If using any of Russia Beyond's content, partly or in full, always provide an active hyperlink to the original material.
Subscribe
to our newsletter!
Get the week's best stories straight to your inbox